
This generates a bigger electrical current. Turning the ignition triggers the acid in the liquid electrolyte solution to react with the active material on the plates (active material refers to any substance in the battery that reacts with the solution to discharge or recharge the battery). A typical lead-acid car battery contains plates of lead alternating with plates made up of other materials, all immersed in an electrolyte solution of about one-third sulfuric acid and two-thirds water. The two types of auto batteries - flooded and AGM batteries - use lead-acid technology.


What the different types of auto batteries are.
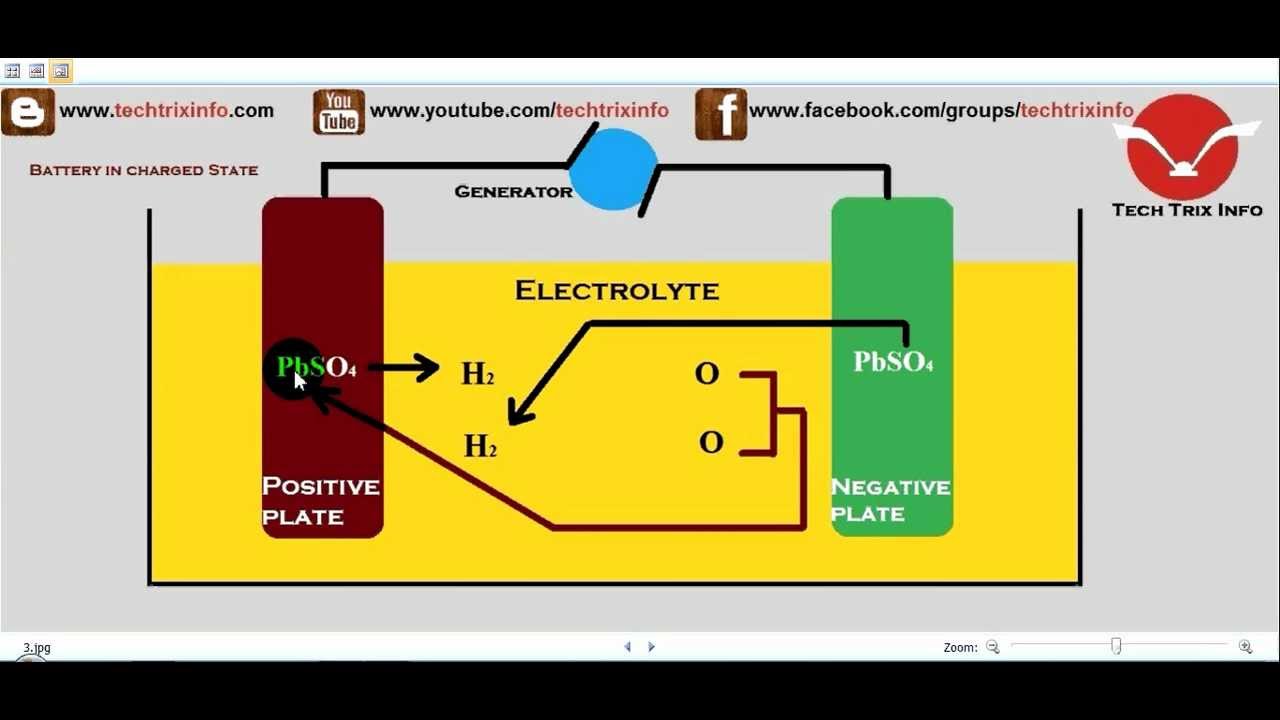
Here’s a simple guide to understanding how car batteries work, from the alternator to cold cranking amps to different types of car batteries.


 0 kommentar(er)
0 kommentar(er)
